One Stray Spark
Click here for a printer-friendly version of this pageMinnesota, the “land of 10,000 lakes,”2911 is the largest brooding area for aquatic birds in the United States. More wild waterfowl hatch there every year than anywhere else in the country.2912 In addition, it’s a central flyway for migratory waterfowl flying south from Canada in the fall.2913 Minnesota also happens to be the nation’s number-one turkey-producing state.2914 That combination gave the state the dubious distinction of the avian influenza “capital of the world.”2915
No wild turkey has ever been found infected with bird flu in Minnesota or elsewhere.2916 Land-based birds, such as turkeys and chickens, are aberrant hosts for the virus.2917 Only when confined in unnatural concentrations does it appear that they can support viral spread that requires close contact. As was shown in pigs, laboratory bird flu transmission studies on turkeys have concluded that “transmission rate is markedly reduced when birds are not confined closely.”2918 In industrial confinement, each turkey is typically each allotted only three square feet of living space.2919 In the wild, turkeys may range over several square miles a day.2920 They naturally congregate in flocks of 10 to 20.2921 Typical confinement facilities house 15,000 turkeys per shed,2922 and those penned outdoors reach flock sizes of 100,000.2923
With so many animals—albeit outdoors—crowded together under skies with so many ducks, scientists have counted more than 100 separate introductions of low-grade bird flu viruses into commercial Minnesota turkey flocks since the 1970s.2924 Yet, even with outdoor flock sizes as large as 100,000 birds, in the sun and open air, not a single one of these viruses mutated into a highly pathogenic strain. High-path viruses have never been known to arise in outdoor chicken or turkey flocks.2925
With tens of millions of ducks2926 excreting tens of billions of viruses, it’s inevitable that outdoor turkey flocks will be caught in the fly-by crossfire. But because they are not intensively confined in the damp, poorly ventilated, and unsanitary sheds, the viruses can’t seem to mutate into highly pathogenic forms. The free-range turkeys rarely suffer serious illness.
Even if the low-path viruses don’t cause sympsoms, though, the bodies of the infected turkeys mobilize resources to knock out the virus, which can result in slower weight gain or reduced egg laying, both of which present a “serious economic burden to turkey producers.”2927 So, starting in the late 1990s, Minnesota turkey farmers followed Canada’s example and converted their free-range or “semi-intensive” farms to industrial confinement operations.
As in Canada,2928 the number of avian influenza infections dropped dramatically.2929 This may have saved the industry money in the short term, but the bill for its shortsightedness may soon fall due. Dave Halvorson is an animal scientist and avian health specialist at the University of Minnesota. In a Poultry Digest review, “Avian Influenza Control in Minnesota,” Halvorson wrote, “If exposed to avian influenza, those range turkeys don’t usually suffer ill effects. But a nonpathogenic influenza virus, when it gets into a confinement situation, causes severe economic loss in morbidity, mortality and body weight loss.”2930 A highly pathogenic virus born of confinement may pose a human health threat as well.
In Monster at Our Door, Mike Davis proposes an analogy for the roles played by indoor and outdoor poultry. “In an epidemiological sense,” he wrote, “the outdoor flocks are the fuse, and the dense factory populations, the explosive charge.”2931 Birds raised outside undoubtedly have a greater exposure to aquatic birds and their viruses,2932 but the buck seems to stops there. If there were only outdoor birds, influenza viruses would rarely have the opportunity to turn highly pathogenic.2933 The co-existence of free-range flocks alongside intensive confinement operations, though, allows for the lit fuse to cause damage. Potential mixing at live poultry markets or via contaminated equipment or clothing could transmit low-grade viruses into confinement facilities, and an epidemic may explode.
What the turkey farmers have been trying to do is get rid of the fuses. By eliminating outdoor production, they hope to eliminate the possibility that some neighboring producer might track some free-range manure into their local feed store. By confining turkeys indoors, there may be fewer fuses—but there is much more to detonate. With that much TNT lying around, all it takes is a spark.
In the three years following the Minnesota decision to move all of the free-range turkeys indooors, more than 25 flocks still came up positive for bird flu.2934 Biosecurity is never absolute. A low-grade strain of H5N1 was even found in a 28,000-bird turkey flock across Lake Superior in Michigan in 20022935 —a year before its Asian cousin H5N1 renewed killing people in Hong Kong.2936 Since turkeys may be more susceptible to infection than chickens,2937 the world is fortunate that turkeys are, as of yet, not raised commercially in China.2938 The bird flu viruses discovered in U.S. turkey sheds were detected in the low-path stage, but had they not been caught in time, some of them might have mutated into widespread killers.
The Food and Agriculture Organization of the United Nations understands that the probability of low-grade infection is higher for outdoor flocks, particularly those not “cooped up and fenced in,”2939 but that the potential detrimental impact of infection within industrial operations is greater.2940 The FAO elaborates in its FAQ, Questions and Answers on Avian Influenza:
[C]hicken to chicken spread, particularly where assisted by intensive husbandry conditions, promotes the virus to shift (adaptation) to more severe type (highly pathogenic type) of infection…. Intensive production conditions favour rapid spread of infection within units and “hotting-up” of virus from low pathogenicity to a highly pathogenic types.2941
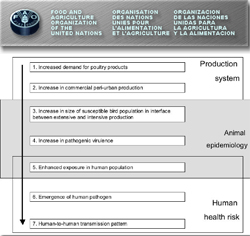
In the FAO diagram above, the evolution of a pandemic is traced, starting with “Increased demand for poultry products” and ending with a bird flu virus capable of human-to-human transmission.2942
Minnesota turkeys have been getting fewer sniffles indoors, but turkey producers may be tempting fate. All it takes is a stray spark—a footprint of duck dropping carried in by a rat—and the situation could explode. Just as bullets seem to find their way into unloaded guns in the household, influenza viruses littered across the countryside seem to find ways into “biosecure” sheds warehousing birds. The industry might do better to disarm completely. To prevent the future emergence of exceptionally deadly viruses like H5N1, the global poultry industry may need to reverse its course of rushing toward greater intensification.
What should poultry producers do right now, though, given that a virus like H5N1 has already been born and is evidently winging its away across Europe? The question remains unanswered, according to veterinarian Karen Becker, senior health adviser within the U.S. Department of Health and Human Services public health emergency preparedness division.2943 Some countries like the Netherlands have placed temporary roofing over outdoor poultry yards on migration routes;2944 others have taken the controversial step of forcing all poultry flocks indoors.2945 Ironically, a subsequent outbreak of duck plague in Germany was blamed on the “severe stresses” associated with confining free-range flocks.3187
The organic lobby in Europe has been most vocal in resisting moves to lock outdoor flocks inside. The Soil Association, the U.K.’s leading organic certifier, argues that disease outbreaks should instead be “minimized by the avoidance of dense stocking level or intensive housing and the promotion of positive animal health through good husbandry and free-range conditions.”2946 It’s true that keeping birds in smaller numbers and densities with access to clean pasture may provide a boost to the birds’ natural immune systems, but, presumably because of prior intensive production in Asia, half of the world is now facing a unique situation in which an avian influenza virus that has already become highly pathogenic could rain from the sky.
H5N1 is not done mutating, though. z+ H5N1 is not a pandemic virus. It would still need to change in order to acquire easy human-to-human transmissibility. That final series of mutations is less likely to happen in someone’s backyard flock of ten birds. Put H5N1 in a 20,000-chicken shed floored with feces, though, and H5N1 might rapidly ratchet up adaptations to the “human-like” epithelium lining the chicken’s respiratory tract and be greatly amplified to further spill back out into the environment. While free-grazing ducks on flooded rice fields in countries like Thailand may be contributing to the spread of H5N1 through water contamination,2947 bringing pasture-raised poultry indoors in Europe may do more harm than good, confining millions more birds in conditions that may most effectively mutate the virus further.
Presumably the only way to significantly slow H5N1 at this late stage is to stop repopulating the sheds. Meat chickens reach slaughter weight so quickly that if breeders stopped breeding and sending chicks out from the hatcheries, every broiler shed in Europe would be vacant in a matter of weeks. That would mean five billion fewer opportunities this year for H5N1 to mutate. Instead we keep reloading.
Perry Kendall is chief medical officer of health for British Columbia and co-chair of the Pan-Canadian Public Health Network.2948 He was there during the 2004 Canadian outbreak, which currently stands as the worse outbreak in North American history, leading to the destruction of 19 million birds. He witnessed that birds kept indoors were more vulnerable than those kept outdoors. “You’ve got 10,000 birds all in a small shed, packed in together—they act like a Petri dish,” Kendall explained in an interview. “The intensely farmed birds tend to be very genetically similar. The methods of farming result in them being actually more frail and more vulnerable to diseases, particularly since there are so many of them in such a small volume (space).” He noted how easy it remains for farm staff to trample virus indoors or for a tractor to spread it from farm to farm. “You need to ramp up your biosecurity level to what you see in a laboratory,” he said, “if you really want to keep infections out of the barns.”2949